Global population kept their eyes on South Korea’s response to the COVID-19 pandemic. Its success illuminated some of the country’s strengths: public health policy, a notably collaborative governing approach and abundant data infrastructure, and a watertight management system of ‘3T formula — Test, Tracing, and Treatment’.
Thanks to the global attention, the South Korean medical industry is becoming competitive on the global stage. However, when it comes to high-end products, the country imports 63% of medtech products from overseas countries, mainly from the United States, Germany, and Japan. The South Korean government attempts to alchemize this moment to boost the country’s medical industry. Government-led support is likely to enhance the opportunities by bolstering clinical and high-tech medical research, combining biotech, medtech, nanotech, and artificial intelligence.


The Daegu-Gyeongbuk Medical Innovation Foundation is one of the R&D hubs of the high-tech medical industry in South Korea. Around one million square meter of land in Daegu was designated for such a research cluster in August 2009. It aims to build strong infrastructure for development of advanced high-end medical products and drugs and create a community between players in medical sectors so that it can help vitalize the local medical industry.
There are four main laboratories: New Drug Development Center, Medical Device Development Center, Laboratory Animal Center, and Drug Manufacturing Center. The drugs being researched in the New Drug Development Center are targeted to cancer, metabolic and cognitive nervous system disorders. Medical Device Development Center is to support product design, prototype production, and functional assessment and it supports IT-centered, image-based convergence diagnosis and therapeutic medical device development. The center also provides ‘One-Stop Total Solution’ which is a commercialization service. Laboratory Animal Center provides customized animal experiment systems and Drug Manufacturing Center supplies the clinical test materials in compliance with the global GMP (good manufacturing practice) standards for pharmaceutical companies and research institutes. The Communication Center in the cluster assists the research and commercialization with its own strategy and planning. Furthermore, its fifth laboratory, Clinical trial Center, is under construction now.
The district surrounding the DGMIF and other one hundred of research institutes such as Korea Brain Research Institute (KBRI), and Laboratory Animal Resources Bank, is called ‘Medivalley.’ It contains its vision to become a medical cluster that creates a way of developing new high-tech medicines and medical equipment. Firms which move in the valley can accept some incentives including regulatory exceptions, financial assistance and tax incentives. The Daegu city is also prolific with about 80,000 young talents graduating every year from 48 colleges and specialized education institutes. DGMIF is still searching for top researchers to lead the development of the high-tech medical industry.
Here below are seven promising companies whose research institutes are located in Daegu-Gyeongbuk Medical Innovation Foundation.
Safe and effective sterilizer using plasma: LowTem Inc.
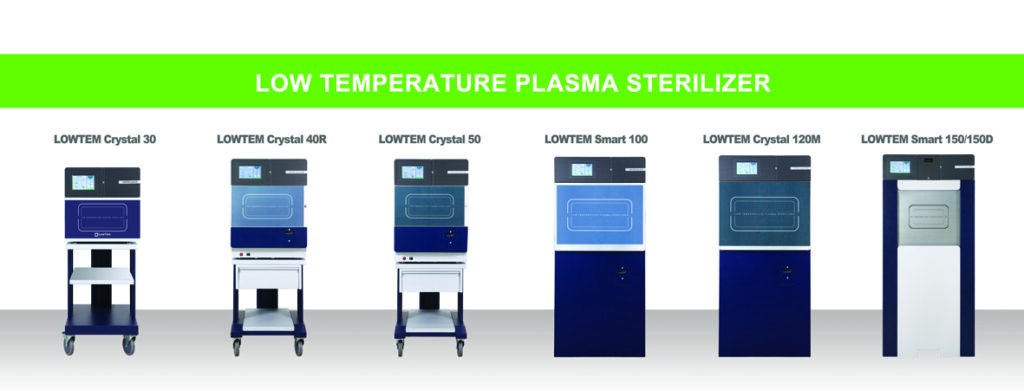

During medical and scientific operations, it is crucial to maintain equipment clean and uncontaminated. Recent studies have found that plasma is effective enough to fully sterilize equipment. Utilizing plasma to achieve sterilization at a low temperature is an alternative to conventional sterilization methods which use ethylene oxide or formaldehyde as far as sterilization of heat-sensitive materials or complicated equipment.
Scientists explain that there are three fundamental states of matter: solid, liquid, and gas. When a solid is heated, it transforms into a liquid and then from a liquid into a gas. Plasma, an ionized gas, is the fourth state of matter. When a gas becomes a plasma, it becomes an unstable state of matter in which the number of electrons are increased or decreased, producing ions. It generates a chemical reaction which can deactivate pathogens in addition to degrading toxins. Hydrogen peroxide (H₂O₂) is used as a disinfecting agent for oxidation. At the point liquid hydrogen peroxide, being dispersed inside the chamber in the sterilizer, becomes to successively gas and plasma by heating up, it oxidizes all microorganisms on the load and inactivates microbial pathogens — such as bacteria, viruses, and fungi — on the surface of medical and dental devices in the chamber.
Hydrogen peroxide plasma has the serious advantage of safety because it does not leave any chemical residues or toxic fumes. The by-products after sterilization are water and oxygen, which is also safe for the environment. One more benefit from using hydrogen peroxide plasma is short aeration time. Its average running cycle is from 30 to 60 minutes, depending on the size of the sterilizers and size and contents of the load.
LowTem Inc. in South Korea is an enterprise specialized in the low temperature plasma sterilization technology. Its own patented technology, ‘Rapid Warm-up and Dry System’, allows to overcome the humidity-related problem, removing residual moisture and the chamber’s cold point. LowTem Series are designed safe and convenient for users to use by providing convenient functions such as automatic PM alarm system, history memory and monitoring system, built-in thermal printer, and printout with actual cycle information. It also warms up within 15 minutes after main power is on, so that standby power consumption is zero during off-duty or overnight. LowTem Series are actively sold in about 40 different countries including Germany, Indonesia, Portugal, and Vietnam. The company is preparing for FDA registration to enter into the sterilization market in the United States.
Company that specialized in dental medical device: Surgident Co., Ltd


Implants are also known as artificial teeth or the third teeth. It is a dental procedure that restores the function of natural teeth by planting a biocompatible implant body in the jawbone that has sufficiently expanded to cover the jawbone through additional surgery such as bone transplantation and bone kidney surgery. The bone modification process appears on the jawbone around the implant after osseointegration, which is a morphological, physiological, and direct bond with the surface of the implant body, where normal functions are maintained.
There are many types of implants, but in recent years, screw-type intra-bone implants are mostly used. Classification by implant type is advantageous for implant stability due to the threaded implant: the initial holding force is increased and the contact area with the bone is large, and the implant is also advantageous for the dispersion of the interlocking load. It is currently the most preferred form. Cylindrical implant, as another type : Stability is also obtained on the surface of the implant fixture and retention resulting from structural differences in jawbone and implant diameter. Related treatments include dentures, bridges, bone grafts (old bone grafts, oral bone grafts), osteoid regeneration, alveolar cleavage, osteogenesis, alveolar enlargement, mitral resection, vitreous grafts, subcutaneous tissue grafts, pedicle grafts.
Dental care is really complicated and diverse, as above mentioned, and it requires a variety of equipment and devices. Surgident Co., Ltd. is a company that manufactures and develops a variety of devices in the dental sector. It already has a branch office in North America and has established various specialized equipment, which shows how professional and diverse dental medical device production and manufacturing of dental medical devices are. You can have a look at them on its website (www.surgident.co.kr). Duk-soo Huh (CEO of Surgident) was awarded ‘a proud small business’ as an excellent manager of Korea by rapidly growing the company based on its solid manufacturing technology. It’s such a professional piece of equipment that I’d rather have experts see it directly through the website than hesitate to mention it.
New Hope for Incurable Neurological Disorder: Astrogen Inc.

An incurable neurological disorder is a disorder in which the incident population is under 20,000, or the incidence rate under 43 percent per 100,000 people. It is unknown and treatment is not established, which can lead to severe disabilities without treatment. There are currently many patients who give up because there is no special treatment for incurable neurological diseases such as tertiary neuralgia, tinnitus, hearing loss, pain after spinal surgery, dizziness, etc. These intractable neurological diseases are difficult to treat with simple neuroblocking, and it is important to recover nerve function or choose the appropriate treatment by judging various situations such as medication, physical therapy, injection therapy, and surgery. But I think it’s called intractable because I’m not sure of the effect yet.
Astrogen Inc. (www.astrogen.co.kr) conducts domestic and international research to find various effective substances that have clinical effects on incurable neurological diseases and develop them as First-in-class drugs, knowing that there is no effective treatment for family members. The effective substances currently under development were directly involved in nerve cell growth and development, protecting nerve cells from oxidative stress, improving mitochondrial function, and showing excellent immune activity. In addition, safe results were obtained in drug interference, cytotoxicity, and genotoxicity experiments, and animal experiments, and were confirmed that cognitive ability improved. In the future, the company plans to successfully complete phase 2 of clinical trials on AST-001, which is targeted to treat Autism Spectrum Disorder (ASD) and gradually expand its scope to develop treatments for other intractable neurological diseases such as Alzheimer’s, Parkinson’s, and Huntington’s by securing excellent new substances as well as pipelines. In some ways, it is a difficult process for the immune system to study and clinicalize unknown causes for as long as the creation of vaccines in the corona crisis.
Incore Co., Ltd’s COVID-19 Sampling Kit used Globally to fight the Pandemic
South Korea is known as a model management country for its most active response since the corona crisis. Inside, the government’s management is important, but it can be seen that small and medium-sized companies are united in prioritizing the safety of the people through participation and donations. It also represents the proven ability to produce and manufacture well-known devices that can be substantially inspected apart from simple donations. As a result, many foreign countries import devices accompanied by Korea’s superior manufacturing technology.
Corona-sampling kit manufactured by Korea-based company, Incore Co., Ltd, obtained overseas certification from the U.S. FDA, Europe CE, and the British NHS and was applied to Nepal for the first time in Korea through KOICA under the Ministry of Foreign Affairs last year. The company also explained that it is currently exporting to six countries, including the UK, the United States and Canada, and that the containers developed this time are stable because they are developed using an electronic sterilization system. Dong-tak Kim, CEO of Incore Co., Ltd., said, “I hope that the sample collection kit, which was developed hard at a time when we are suffering from the prolonged corona 19, will be of little help to prevent the spread of corona19.”
The research institute of Incore Co,.Ltd. is located in a region called Daegu in Korea and seems to stand out in the Daegu Medivalley. The agency recognises and cooperates, indicating the potential for further development.The Corona crisis is showing the second stage of the vaccine and building mass immunity through antibodies, but attention should be paid to the encoder that produces safe coronavirus kits because it is such a mutant virus.
Detecting radiation with an advanced sensor: J. S. Techwin

J.S. Techwin is making radiation detectors with sensor and amplifier technology. You may have heard of the huge damage to people and the environment caused by radiation leaks in Japan and Russia, which are already well known. In addition, radiation-based medical care is close to our lives. If we recognize that radiation that only exists in industries and facilities is close to us, we would like to inform you of J.S. Techwin’s portable detector and (real time dosimeter).
The company seems to be specialized in consumer, industrial, and medical manufacturing and is researching and developing core sensors for radiation detectors. Currently, radiation detectors are difficult to detect instantaneous exposure, which means that the response using GM tubes is slow, so measurements are made for 20-30 seconds to indicate the lateral value. As a result, there seem to be many loopholes that can be exposed to radiation several times. For example, some products have a narrow measurement width, and when large radiation is exposed, they are not detected and caught at all.
Jun-seok Suh graduated from Seoul National University in Korea and studied nuclear physics in the United States and Germany. He worked at CERN (European Organization for Nuclear Research) in Geneva, Switzerland and Brookhaven in the U.S. for 11 years and was a professor at Kyungpook National University in South Korea. Based on this long experience, he intended to manufacture Positron Emission Tomography (PET) directly. PET is a method of nuclear medical testing that allows physiological, chemical and functional images of the human body to be presented in three dimensions using radioactive drugs that emit positrons. Currently, it is mainly used to diagnose various cancers and is known to be a useful test for distinguishing cancer, setting up weapons, evaluating recurrence, and determining treatment effects.
Strong player in the plasma sterilization market: Plasmapp Inc.

Microorganisms such as bacteria, fungi, and viruses cause diseases. Not all microorganisms can be successfully sterilized in a steam sterilizer or an autoclave. Several reports suggest that plasma, an ionized gas which represents the fourth state of matter, can essentially inactivate them. Accumulated scientific knowledge has led people to be aware of the utility of plasma technology for disinfection and improved the efficacy of sterilization against various microorganisms.
Plasmapp Inc. is a venture enterprise which started in a laboratory in Korea Advanced Institute of Science and Technology (KAIST). It develops and manufactures medical devices and industrial equipment by utilizing its own plasma technology.
Applied with the world’s first direct injection technology, STERLINK, a low-temperature plasma sterilizer, is designed to have a vacuum sealing pouch in it. The pouchpack named ‘STERPACK’ also enables checking real-time sterilization conditions and using safe disposable sterilization cassettes. With STERLINK, you can disinfect your medical apparatus in only seven minutes. The importance of its safety cannot be overstated because utilizing plasma at low temperature does not produce any toxin residues or damage complicated equipment. It is on sale all across the globe including 60 countries with acquisition of diverse certification.
There are two more main products of the company: ACTLINK which disinfects, regenerates and activates dental and orthopedic implant surfaces, and Linear Jet Plasma Source (LJPS) which is a general industrial plasma and can be applied to secondary battery separators. ACTILINK in two types into dental and orthopedic is in its final stages of development and will be completed and start sales within the latter half of this year.
Plasmapp Inc, established in 2015, is posting more than 70 percent of its sales through overseas exports and plans to cooperate with more global companies. The company is currently taking steps with the goal of listing on KOSDAQ next year.
Effective Rehabilitation Products and Services: HUCASYSTEM Inc.


Europe has dominated the rehabilitation equipment market for many years. In 2019, Europe accounted for the largest share of the market. Factors such as supportive government initiatives for safe patient handling, rising geriatric population, and the high burden of chronic conditions drove the growth of the European market, according to the report in 2020 from global research company, ‘Markets and Markets’. On the other hand, the report also shows that the Asia Pacific market is projected to register the highest CAGR during the forecast period with the consideration of the growing prevalence of chronic conditions, increasing venture capital investments, availability of technologically advanced products, and strategic expansion of market players in this region.
It is also expected to see a new player from South Korea, HUCASYSTEM Inc., in the market. Founded in February 2019, HUCASYSTEM Inc. provides rehabilitation products and services for people suffering from neurological movement disorders by harnessing advanced robot technology. The company’s primary goal is to play a leading role in resolving the rapidly increasing social and economic problems of the disabled with the aging of the population.
Entry-level Gait Rehabilitation Robot System (GTR-S), which is a major product of HUCASYSTEM, can perform a two-stage gait protocol — learning and training — and can expect aerobic exercise effects. The second major product, Complex Upper & Lower Limbs Gait Rehabilitation Robot System (GTR-A), can perform a three-stage gait protocol — learning, practice, and training — and can expect higher aerobic exercise effects. Moreover, the company has acquired a Class ǁ medical device manufacturing certification and approval for both devices by the Ministry of Food and Drug Safety of Korea.
In the future, HUCASYSTEM will launch a project to establish a rehabilitation service platform, named ‘HUCAVERSE’, linking three parties — medical institutions, rehabilitation centers, and individuals — with its own technology and solutions in order to provide systematic management and high-quality rehabilitation services. Furthremore, it maps out its schemes to build a K-Rehab care network that allows patients to receive professional health and medical care services at home without visiting medical institutions. The company is expected to grow into a high-tech rehabilitation company and expand its product line-up with pediatric gait rehabilitation robots and gait exercise robots soon.
Kayla Hong
Asia Journal

